HBV Assets
Pursuing a functional cure for chronic hepatitis B (cHBV).
Chronic hepatitis B (cHBV) is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). Persons chronically infected with HBV are at increased risk of developing significant liver disease, including cirrhosis, or permanent scarring of the liver, as well as liver failure and hepatocellular carcinoma (HCC). Given the widespread prevalence of cHBV infections and the shortcomings of existing treatment regimens, we believe there is a compelling market opportunity for an HBV curative regimen.

HBV by the Numbers
>250
million people
suffer from cHBV worldwide.
2
million people
suffer from cHBV in the United States.
1.1
thousand mortalities
occur due to cHBV related complications each year.

Coronovirus
Lifelong therapeutics, and the hope for more.
Today’s current treatment options for cHBV include pegylated interferon-α (Peg-IFNα) and nucleos(t)ide analogues (NAs). However, in most cases, once Peg-IFNα and NA therapies are stopped, virus replication resumes, and liver inflammation and fibrosis may still progress. While these treatments reduce viral load, less than 10% of patients are functionally cured (defined as sustained HBsAg loss and HBV DNA <LLOQ 24 weeks off-treatment, with or without anti-HBs) after a variable and usually prolonged treatment duration. With such low cure rates, most patients with cHBV are required to take NA therapy daily for the rest of their lives.
Despite these treatment options, there remains an unmet medical need for novel, finite, and more efficacious cHBV treatments that result in improved long-term outcomes, including an increased chance for functional cure.
Pursuing a cure for HBV through multiple, complementary mechanisms.
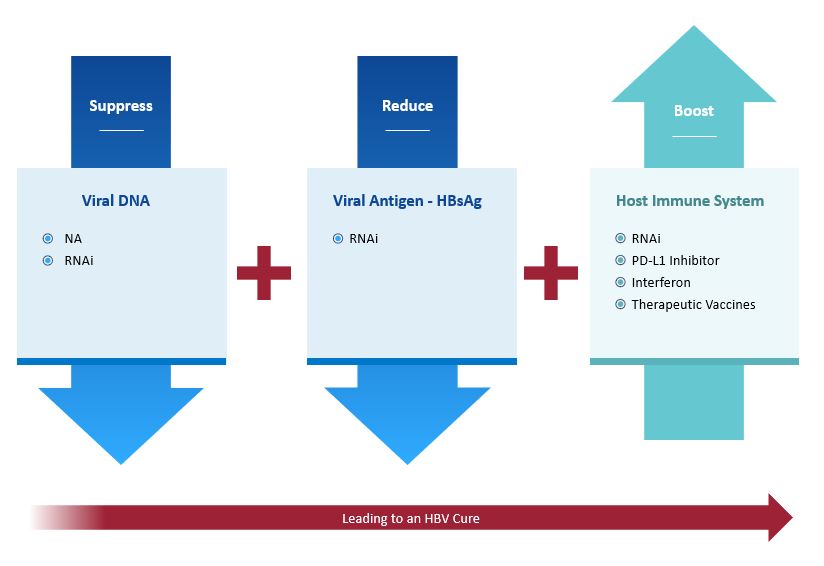
Recognizing that monotherapy approaches are unlikely to be successful in promoting functional cure in patients with cHBV, we are taking a three-pronged approach that combines compounds with complementary mechanisms of action to suppress viral DNA, reduce viral antigens and boost the host immune system.
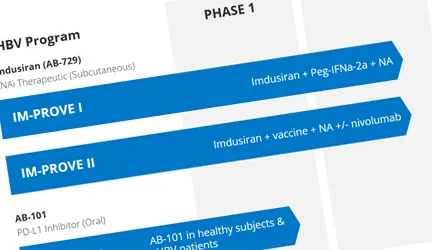
Our lead asset, imdusiran (AB-729), an RNAi therapeutic, is being developed as the cornerstone in a combination treatment strategy that includes NA therapy and potentially our proprietary compound AB-101 (PD-L1 inhibitor) and/or Peg-IFNα, and other compounds in development or approved.
Here’s how it works.
Suppress viral DNA
Block replication via NA, RNAi.
Reduce viral antigens
Block HBsAg via RNAi.
Boost immune response
Induce immune modulation via PD-L1 inhibitor, interferon, therapeutic vaccines, or RNAi.
3-Pronged Approach to Therapeutic Success